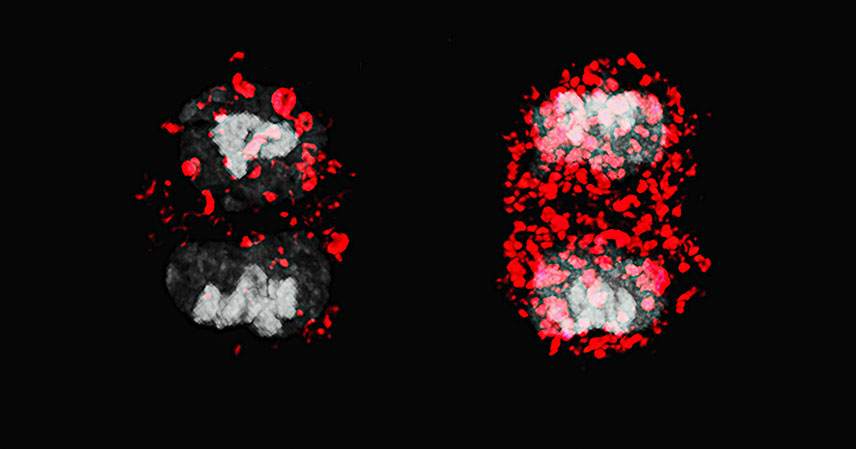
Our immune system is a marvel of biological engineering. At its core are specialized cells, like the formidable killer T cells, responsible for identifying and eradicating infected or cancerous cells. A critical process enabling their effectiveness is asymmetric cell division (ACD). This fundamental biological mechanism ensures that when a killer T cell divides, its two daughter cells are not identical. Instead, they inherit different cellular components, leading them down distinct developmental paths.
This remarkable process is essential for generating a comprehensive and long-lasting immune response. It allows the body to simultaneously mount an immediate defense and prepare for future encounters with the same pathogen. Understanding this intricate cellular dance offers profound insights into how our bodies maintain health and fight disease.
The Intricacies of Asymmetric Cell Division (ACD) in T Cells 🧬
Asymmetric cell division is a sophisticated cellular strategy. When a killer T cell encounters a threat, it begins to proliferate rapidly. However, these divisions are not merely about increasing cell numbers. During ACD, the dividing T cell distributes its internal components unevenly between the two new cells.
One daughter cell typically receives a set of components that primes it to become an effector T cell. These are the immediate responders, short-lived but highly potent fighters. They are programmed for direct combat, swiftly eliminating infected cells. Their primary mission is to clear the current infection.
The other daughter cell, inheriting a different cellular cargo, develops into a memory T cell. Memory T cells are the immune system’s long-term protectors. They persist in the body for extended periods, sometimes decades. Should the same pathogen reappear, these memory cells can quickly reactivate and mount a faster, more robust immune response. This dual outcome is crucial for adaptive immunity.
The Critical Balance: Effector vs. Memory T Cells ⚖️
The generation of both effector and memory T cells through ACD is vital for robust immunity. Effector T cells provide the immediate firepower needed to control an ongoing infection. Without them, the body would struggle to clear pathogens effectively. Their rapid action prevents widespread damage and disease progression.
Conversely, memory T cells are the foundation of immunological memory. They are why vaccines work and why we often gain lifelong immunity after certain infections. These cells ensure that future encounters with the same threat are met with an accelerated and powerful defense. The precise balance between these two cell types is meticulously regulated. Any disruption could compromise the immune system’s ability to protect the host. For example, in chronic infections or cancer, T cells can become exhausted, impacting the generation or function of these critical subsets.
Implications for Immunology and Therapeutic Development 💡
The discovery and deeper understanding of ACD in killer T cells hold immense promise for medical science. Researchers believe that by manipulating this process, it might be possible to enhance immune responses. This knowledge could revolutionize how we approach various health challenges.
For instance, in vaccine development, insights into ACD could help design more effective vaccines. Scientists could potentially optimize conditions to favor the generation of more long-lived memory T cells. This could lead to vaccines that offer stronger and more durable protection against infectious diseases. Imagine vaccines requiring fewer booster shots or providing broader immunity.
Furthermore, this research has significant implications for cancer immunotherapy. Many current cancer treatments aim to unleash the immune system against tumors. If we can control T cell fate during expansion, we might be able to generate more potent and persistent effector T cells. This could help overcome T cell exhaustion, a common challenge in treating solid tumors. It might also improve the efficacy of CAR T-cell therapies, making them more durable.
Understanding ACD may also shed light on autoimmune diseases. Dysregulation in T cell differentiation could potentially contribute to conditions where the immune system mistakenly attacks healthy tissues. Future research could explore whether modulating ACD could help restore immune balance in these patients. This area of study is still emerging, but the potential is vast.
Key Insights ✨
- Killer T cells undergo asymmetric cell division (ACD) to produce two distinct daughter cells.
- One daughter cell becomes a short-lived effector T cell, responsible for immediate pathogen clearance.
- The other daughter cell develops into a long-lived memory T cell, providing sustained protection against future infections.
- This process is fundamental for establishing both immediate defense and long-term immunological memory.
- Understanding ACD could lead to advancements in vaccine design and more effective cancer immunotherapies.
The intricate dance of asymmetric cell division within killer T cells highlights the sophistication of our immune system. This fundamental biological mechanism is a cornerstone of our body’s defense strategy. While research continues to uncover its full complexities, the potential applications are truly exciting. Further studies may unlock new avenues for treating diseases and enhancing human health. This ongoing exploration promises to deepen our understanding of immunity itself.
Source: What determines the fate of a T cell? Research highlights cellular ‘housekeeping’ mechanism