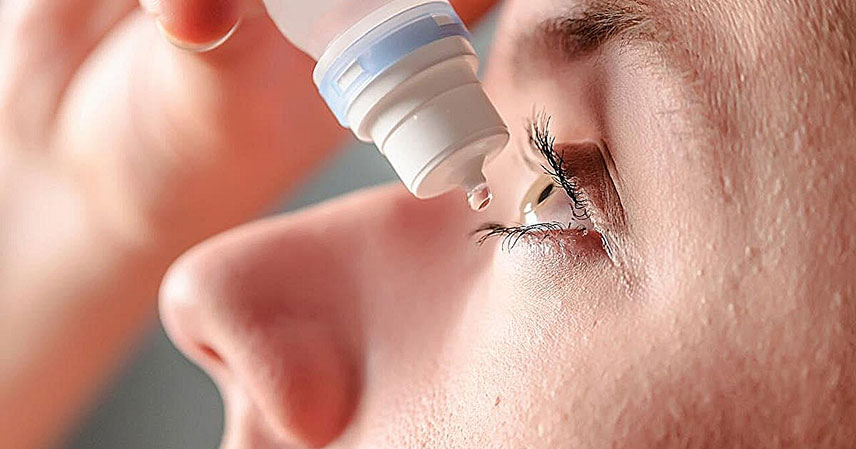
The U.S. Food and Drug Administration (FDA) recently announced a significant approval. They have cleared Yuvezzi (carbachol and brimonidine tartrate) ophthalmic solution 2.75%/0.1% for adults. This eye drop targets presbyopia, a common age-related vision condition. It marks a notable advancement as the first and only dual-agent eye drop for this purpose.
This approval introduces a novel therapeutic option. It offers a new approach for millions of individuals. These individuals struggle with diminishing near vision as they age. The development of Yuvezzi, previously known as Brimochol PF, could significantly impact daily life for many.
Understanding Presbyopia: The Age-Related Vision Challenge 👓
Presbyopia is a natural part of the aging process. It typically begins around age 40. The eye’s natural lens hardens and loses flexibility. This makes it difficult to focus on close-up objects. Common symptoms include blurred near vision and eye strain. Many people find themselves holding reading material further away.
Globally, hundreds of millions of people experience presbyopia. It affects almost everyone eventually. Traditional solutions often involve corrective lenses. These include reading glasses or bifocals. Some individuals opt for multifocal contact lenses. Surgical interventions like refractive lens exchange are also available. However, these options come with their own considerations, including convenience and invasiveness.
The impact of presbyopia extends beyond vision. It can affect daily tasks. Reading, using a smartphone, or working on a computer become challenging. This condition often leads to frustration. It can also reduce productivity. Finding a convenient and effective treatment is therefore highly sought after.
Yuvezzi’s Novel Approach: The Science Behind Dual-Action 🔬
Yuvezzi distinguishes itself through its unique formulation. It combines two active pharmaceutical ingredients: carbachol and brimonidine tartrate. This dual-agent approach is what makes it a first of its kind. Each component plays a specific role in improving near vision.
Carbachol is a cholinergic agonist. It works by causing the pupil to constrict. This effect is known as miosis. A smaller pupil increases the depth of focus. This allows for clearer vision at close distances. It essentially creates a pinhole effect, which helps to sharpen images.
Brimonidine tartrate is an alpha-2 adrenergic agonist. Its primary role in this formulation is multi-faceted. It also contributes to pupil constriction. Furthermore, it may help to mitigate potential side effects associated with miotics. This combination is designed to work synergistically. It aims to provide effective and well-tolerated near vision improvement. This innovative combination represents a significant step forward in ophthalmic pharmacology.
The development of a dual-agent eye drop suggests a sophisticated understanding of the eye’s mechanics. Researchers aimed for an optimized balance. They sought maximum efficacy with minimal adverse effects. This careful formulation is crucial for patient compliance. It also ensures long-term usability. The FDA’s approval underscores the potential of this combined therapy.
Implications for Patients and the Ophthalmic Market 📈
The approval of Yuvezzi offers a new, non-invasive option for presbyopia. Patients who dislike wearing glasses or contact lenses may find this appealing. It provides an alternative to constant lens use. This could significantly enhance quality of life. The convenience of an eye drop is a major benefit. It integrates easily into daily routines.
However, it is important to remember that Yuvezzi is not a cure for presbyopia. It is a treatment to manage symptoms. Individuals considering this option should consult their eye care professional. An ophthalmologist or optometrist can determine suitability. They can also discuss potential side effects and proper usage. This ensures the best possible outcome for each patient.
The ophthalmic market is constantly evolving. Yuvezzi’s entry signifies a growing trend. There is increasing innovation in non-surgical vision correction. This approval could spur further research and development. Other pharmaceutical companies might explore similar dual-action or novel single-agent therapies. This competition could ultimately benefit patients. It provides more choices and potentially better outcomes. The market for presbyopia treatments is substantial and continues to grow with an aging global population.
Key Insights from the Approval 💡
- Yuvezzi is the first and only dual-agent eye drop approved by the FDA for presbyopia.
- It combines carbachol and brimonidine tartrate. These work together to improve near vision.
- This approval provides a non-surgical alternative. It helps manage age-related blurry near vision.
- The new treatment could offer enhanced convenience for millions of adults. It may reduce reliance on reading glasses.
- This advancement highlights ongoing innovation in ophthalmic pharmaceutical solutions. It could pave the way for future developments.
Source: FDA approves combination Yuvezzi eye drop for presbyopia in adults