Parkinson’s disease, a debilitating neurological condition affecting millions worldwide, has long puzzled scientists. While the gradual loss of brain cells and the resulting tremors and stiffness are well-known symptoms, the underlying cause has remained elusive. However, groundbreaking research focusing on the behavior of a specific protein may finally shed light on this complex illness and pave the way for earlier diagnosis and potentially new treatments.
Recent studies have shifted the focus from large protein aggregates to smaller, more toxic structures, offering a new perspective on the disease’s progression. This research promises to revolutionize our understanding of Parkinson’s and ultimately improve the lives of those affected.
What Happened? 📝
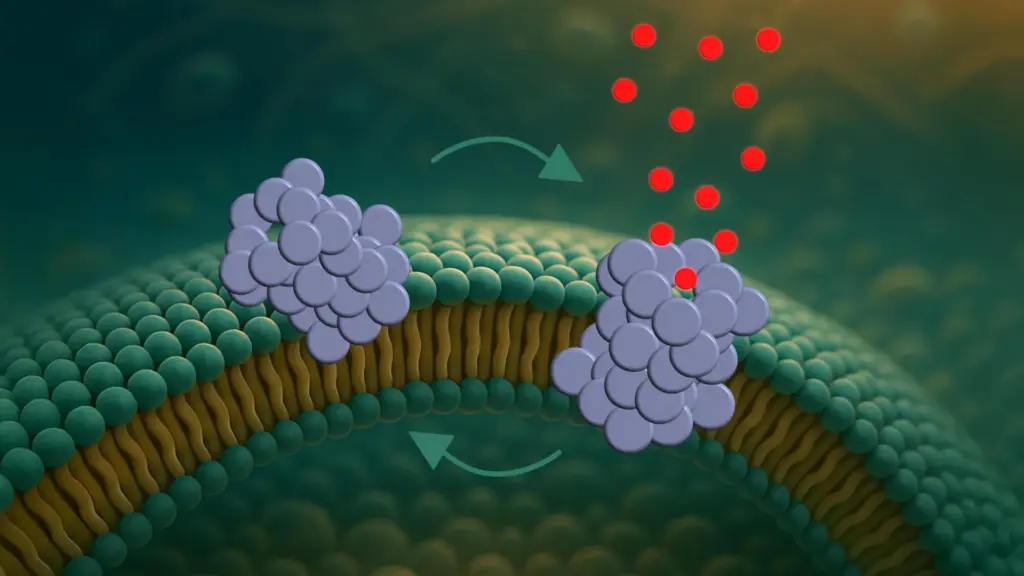
The core of this exciting discovery centers on α-synuclein, a protein involved in healthy brain cell communication. In Parkinson’s, this protein malfunctions, clumping together into toxic structures. While previous research primarily focused on larger fibrils, a new study published in ACS Nano highlights the significance of smaller, more dangerous structures called α-synuclein oligomers.
These oligomers, according to the research, create tiny holes, or pores, in the membranes of nerve cells. These pores are not static; they dynamically open and close, like microscopic revolving doors. This dynamic nature might explain why cells don’t die immediately, as the cell’s own mechanisms may temporarily compensate for the disruption.
How the Discovery Was Made 🔬
This groundbreaking research utilized a newly developed single-vesicle analysis platform. This innovative technology allows scientists to observe the interaction between individual proteins and artificial cell membranes (vesicles) in real-time. It’s essentially like watching a molecular movie in slow motion, providing unprecedented detail into the process of pore formation.
This platform provides a clean experimental environment. Researchers can isolate specific factors, enabling precise measurements and a deeper understanding of the mechanisms involved. The ability to observe these interactions in real-time is a significant advancement, allowing for a more comprehensive analysis than previously possible.
The Role of Oligomers and Pore Formation 🦠
The study meticulously details a three-step process of pore formation by α-synuclein oligomers. First, the oligomers attach to the cell membrane, particularly at curved regions. Next, they partially insert themselves into the membrane. Finally, they form pores that disrupt the cell’s internal balance by allowing molecules to pass through uncontrollably.
The dynamic opening and closing of these pores is a key finding. This dynamic behavior suggests a more nuanced understanding of cell death in Parkinson’s, potentially explaining why the disease progresses gradually rather than causing immediate cell collapse. The ability to observe this dynamic process directly is a major breakthrough.
Testing Nanobodies as Potential Diagnostic Tools 🧪
Researchers have already begun exploring the potential of nanobodies – small antibody fragments – to target these oligomers. Early testing suggests that these nanobodies could be highly selective diagnostic tools, capable of detecting the presence of oligomers at very early stages of the disease. This is incredibly significant, as Parkinson’s is usually diagnosed only after considerable neuronal damage has already occurred.
While the nanobodies did not prevent pore formation in this study, their potential for early detection remains promising. Early diagnosis could lead to earlier intervention, potentially slowing or even preventing the progression of the disease. This is a critical step towards improving patient outcomes.
Mitochondrial Targeting and Future Research 💡
Interestingly, the study reveals that pore formation is not random. The pores tend to appear in specific membrane types, particularly those resembling the membranes of mitochondria – the cell’s energy powerhouses. This suggests that mitochondrial damage may be an early event in the Parkinson’s disease process.
This research, however, was conducted in model systems, not in living cells. The next crucial step involves replicating these findings in more complex biological tissues, where numerous other factors influence the disease progression. The researchers emphasize the need for further investigation in more realistic settings.
Key Takeaways 🔑
- α-synuclein oligomers form dynamic pores in nerve cell membranes, contributing to Parkinson’s disease.
- A new single-vesicle analysis platform allows real-time observation of this pore formation.
- Nanobodies show promise as highly selective diagnostic tools for early detection of Parkinson’s.
- Mitochondrial damage may be an early event in Parkinson’s pathogenesis.
- Further research is needed to validate these findings in living cells and more complex biological systems.
This research represents a significant leap forward in our understanding of Parkinson’s disease. While a cure remains a distant goal, these insights offer hope for new therapies, adding to other recent breakthroughs like the discovery of the protective role of Midkine protein in Alzheimer’s. The ongoing research utilizing the advanced single-vesicle analysis platform promises to unlock even more secrets of this complex disease, ultimately leading to improved treatments and better patient outcomes.
Source: Scientists watch Parkinson’s protein drill holes in brain cells